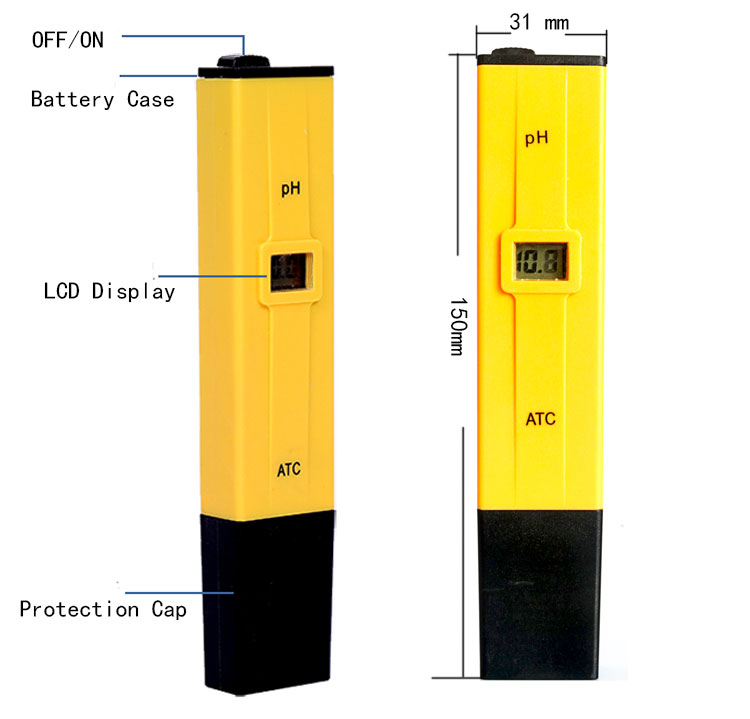
Digital ATC PH Meter PH Tester
Original price was: ₨1,200.00.₨925.00Current price is: ₨925.00.
- 100% Brand New PH Meter With Calibration
- Suitable for any aquarium industry, fishing industry, swimming pool, spa, school laboratory, food and beverage,drinking water,etc.
- Lightweight,compact pH meter.Completely hidden in a bag or pocket for easy daily carrying.
- Easy to use,remove the protective cap and immerse the pH meter electrode in the solution
- Hand-held design,convenience
Description
For Details watch youtube video:
Descriptions:
This ATC PH meter digital pen style pH meter is an ideal instrument for aquarium, fishing industry, swimming pool, school laboratory, food & beverage etc.
This is an economic type ph meter with high precision,digital readout, large LCD display screen, Very convenient to test ph value for lab and industry and other
aspects, because of the cabinet appearance and light weight.
Please calibrate with the buffer solution before use.
Specifications:
Weight: 51g
Excellent PH meter, very accurate and durable
Measuring pH Range: 0.0 – 14.0 pH
Resolution: 0.1 pH
Accuracy: ±0.1 pH (20°C), ±0.2 pH
Temperature Compensation: 0 – 50°C (32-122°F).
Calibration: manual,1 point
Battery:AG 13 button cell batteries (included )
Easy to Calibrate
You can simply follow the instruction on the provided packet of solution 4.00or 6.86 immerse the PH tester in the solution and adjust with the provided screwdriver to dial it in.
Easy to Operation
1. Remove the protective cap.
2. First clean the electrode with distilled water, and suck it with filter-paper.
3. Turn on PH by “ON-OFF”switch located on the top of battery case.
4. Immerse the PH meter electrode in solution up to be the immersion level.Under no circumstances immerse above display level.
5. Stir gently and wait for the reading to stabilize.
6. After use, Switch off PH, Use the distilled water clean the electrode and replace the protective cap.
Principle of operation:
The pH meters measure the voltage between two electrodes and display the result converted into the corresponding pH value. They comprise a simple electronic amplifier and a pair of electrodes, or alternatively a combination electrode, and some form of display calibrated in pH units. It usually has a glass electrode and a reference electrode, or a combination electrode. The electrodes, or probes, are inserted into the solution to be tested.[8]
The design of the electrodes is the key part: These are rod-like structures usually made of glass, with a bulb containing the sensor at the bottom. The glass electrode for measuring the pH has a glass bulb specifically designed to be selective to hydrogen-ion concentration. On immersion in the solution to be tested, hydrogen ions in the test solution exchange for other positively charged ions on the glass bulb, creating an electrochemical potential across the bulb. The electronic amplifier detects the difference in electrical potential between the two electrodes generated in the measurement and converts the potential difference to pH units. The magnitude of the electrochemical potential across the glass bulb is linearly related to the pH according to the Nernst equation.
The reference electrode is insensitive to the pH of the solution, being composed of a metallic conductor, which connects to the display. This conductor is immersed in an electrolyte solution, typically potassium chloride, which comes into contact with the test solution through a porous ceramic membrane. The display consists of a voltmeter, which displays voltage in units of pH.
On immersion of the glass electrode and the reference electrode in the test solution, an electrical circuit is completed, in which there is a potential difference created and detected by the voltmeter. The circuit can be thought of as going from the conductive element of the reference electrode to the surrounding potassium-chloride solution, through the ceramic membrane to the test solution, the hydrogen-ion-selective glass of the glass electrode, to the solution inside the glass electrode, to the silver of the glass electrode, and finally the voltmeter of the display device. The voltage varies from test solution to test solution depending on the potential difference created by the difference in hydrogen-ion concentrations on each side of the glass membrane between the test solution and the solution inside the glass electrode. All other potential differences in the circuit do not vary with pH and are corrected for by means of the calibration.
For simplicity, many pH meters use a combination probe, constructed with the glass electrode and the reference electrode contained within a single probe. A detailed description of combination electrodes is given in the article on glass electrodes.
The pH meter is calibrated with solutions of known pH, typically before each use, to ensure accuracy of measurement. To measure the pH of a solution, the electrodes are used as probes, which are dipped into the test solutions and held there sufficiently long for the hydrogen ions in the test solution to equilibrate with the ions on the surface of the bulb on the glass electrode. This equilibration provides a stable pH measurement.

Additional information
| Weight | 0.15 kg |
|---|---|
| Dimensions | 12 × 10 × 8 cm |
| shape | Blue, yellow |
You must be logged in to post a review.








Reviews
There are no reviews yet.